Nitrogen isotope fractionation during terrestrial core-mantle separation
Affiliations | Corresponding Author | Cite as-
Share this article
![]()
![]()
![]()
![]()
![]()
Article views:12,998Cumulative count of HTML views and PDF downloads.
- Download Citation
-
Rights & Permissions
Abstract
Figures and Tables
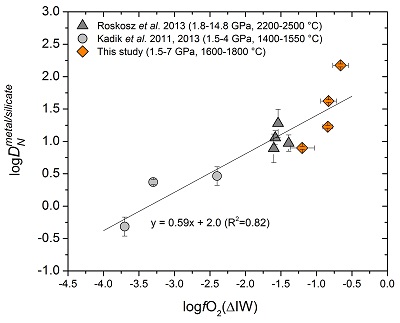
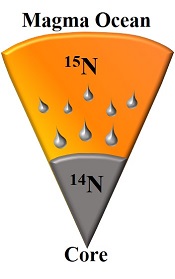
Figure 1 Nitrogen partition coefficients between liquid Fe-rich metal and silicate melt (DNmetal/silicate). The published DNmetal/silicate data (Kadik et al., 2011, 2013; Roskosz et al., 2013) are plotted for comparison. DNmetal/silicate is a function of oxygen fugacity, regardless of pressure or temperature. The slope of the trend line is moderately lower than 0.75 expected based on Eq. 1, which could be due to a small fraction of nitrogen present as N2 in the silicate melt. Errors of DNmetal/silicate in this study are at 95 % confidence interval. |

Table 1 Experimental results on nitrogen partitioning and isotope fractionation between liquid Fe-rich metal and silicate melt. |
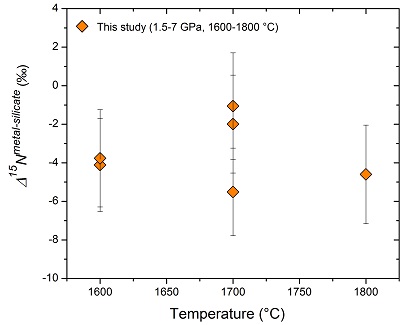
Figure 2 Nitrogen isotope fractionation between liquid Fe-rich metal and silicate melt (Δ15Nmetal-silicate). Temperature has a very limited effect on Δ15Nmetal-silicate in the P-T range studied. Errors of Δ15Nmetal-silicate are at 95 % confidence interval. |
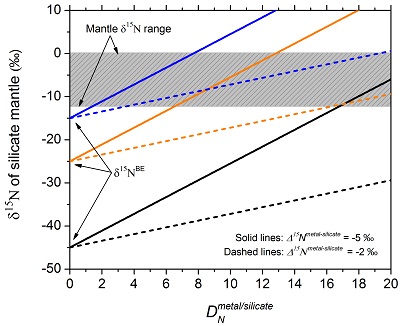
Figure 3 The modelled δ15N value of the silicate mantle as a function of DNmetal/silicate, just after complete core-mantle separation, using the Rayleigh distillation model (Eq. 5) and the relationship between ƒNmantle and DNmetal/silicate (Eqs. 6 and 7). Three different δ15NBE values are used: the lowest value (−45 ‰) is the lowest value observed so far for Earth’s mantle and enstatite chondrites (Grady et al., 1986; Palot et al., 2012); the values of −25 ‰ and −15 ‰ correspond to the average and highest δ15N values of enstatite chondrites (Grady et al., 1986), respectively. Two different values of −2 ‰ and −5 ‰ are used for the dashed lines and solid lines, respectively. The gray box represents the main range of δ15N values observed for the silicate mantle (Marty and Dauphas, 2003; Palot et al., 2012; Cartigny and Marty, 2013), which may be produced from the average nitrogen isotopic composition of enstatite chondrites by core-mantle separation with DNmetal/silicate between 5 and 20. |
| Figure 1 | Table 1 | Figure 2 | Figure 3 |
Supplementary Figures and Tables

Table S-1 Major and minor element contents in silicate melt and liquid Fe-rich metal (in wt. %). |
| Table S-1 |
top
Introduction
Nitrogen isotopes may constrain the origin of terrestrial volatiles (Javoy, 1997
Javoy, M. (1997) The major volatile elements of the earth: Their origin, behavior, and fate. Geophysical Research Letters 24, 177-180.
; Marty, 2012Marty, B. (2012) The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth and Planetary Science Letters 313-314, 56-66.
), as well as the evolution and interaction of different terrestrial reservoirs (Marty and Dauphas, 2003Marty, B., Dauphas, N. (2003) The nitrogen record of crust-mantle interaction and mantle convection from Archean to Present. Earth and Planetary Science Letters 206, 397-410.
). However, the origin and evolution of terrestrial nitrogen isotopes remains poorly understood. The present-day upper mantle δ15N, inferred from fibrous diamonds and mid-ocean ridge basalts (MORBs) is −12 to 0 ‰ and converges towards a globally uniform value of −5 ‰ (Marty and Dauphas, 2003Marty, B., Dauphas, N. (2003) The nitrogen record of crust-mantle interaction and mantle convection from Archean to Present. Earth and Planetary Science Letters 206, 397-410.
; Cartigny and Marty, 2013Cartigny, P., Marty, B. (2013) Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359-366.
). The δ15N value of the organic matter and metasediments is overall positive, and the δ15N value of Earth’s surface (crust + atmosphere) is approximately +2 ‰ (Cartigny and Marty, 2013Cartigny, P., Marty, B. (2013) Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359-366.
; Thomazo and Papineau, 2013Thomazo, C., Papineau, D. (2013) Biogeochemical cycling of nitrogen on the early Earth. Elements 9, 345-351.
). The oceanic island basalts (OIBs), thought to be derived from the lower mantle (e.g., Shen et al., 1998Shen, Y., Solomon, S.C., Bjarnason, I.T., Wolfe, C.J. (1998) Seismic evidence for a lower-mantle origin of the Iceland plume. Nature 395, 62-65.
; Stuart et al., 2003Stuart, F.M., Lass-Evans, S., Fitton, J.G., Ellam, R.M. (2003) High 3He/4He ratios in picritic basalts from Baffin Island and the role of a mixed reservoir in mantle plumes. Nature 424, 57-59.
), have δ15N of −2 to +8 ‰, with a mean value of about +3 ‰ (Dauphas and Marty, 1999Dauphas, N., Marty, B. (1999) Heavy nitrogen in carbonatites of the Kola Peninsula: A possible signature of the deep mantle. Science 286, 2488-2490.
; Marty and Dauphas, 2003Marty, B., Dauphas, N. (2003) The nitrogen record of crust-mantle interaction and mantle convection from Archean to Present. Earth and Planetary Science Letters 206, 397-410.
). However, diamonds derived from the mantle transition zone and lower mantle show similar δ15N values to the upper mantle (Palot et al., 2012Palot, M., Cartigny, P., Harris, J., Kaminsky, F., Stachel, T. (2012) Evidence for deep mantle convection and primordial heterogeneity from nitrogen and carbon stable isotopes in diamond. Earth and Planetary Science Letters 357, 179-193.
). Therefore the positive δ15N values of OIBs were interpreted to be results of addition of recycled sediments to the OIB source region (Dauphas and Marty, 1999Dauphas, N., Marty, B. (1999) Heavy nitrogen in carbonatites of the Kola Peninsula: A possible signature of the deep mantle. Science 286, 2488-2490.
; Marty and Dauphas, 2003Marty, B., Dauphas, N. (2003) The nitrogen record of crust-mantle interaction and mantle convection from Archean to Present. Earth and Planetary Science Letters 206, 397-410.
). Nevertheless, most diamond populations with Archean ages define a δ15N range of −12 to +5 ‰, with a mode around −5 ‰ (Cartigny et al., 2009Cartigny, P., Farquhar, J., Thomassot, E., Harris, J., Wing, B., Masterson, A., McKeegan, K., Stachel, T. (2009) A mantle origin for Paleoarchean peridotitic diamonds from the Panda kimberlite, Slave Craton: evidence from 13C-, 15N-and 33,34S-stable isotope systematics. Lithos 112, 852-864.
), indicating no obvious secular change in mantle δ15N and thus limited nitrogen recycling compared to the nitrogen abundance in the mantle. Li et al. (2013)Li, Y., Wiedenbeck, M., Shcheka, S., Keppler, H. (2013) Nitrogen solubility in upper mantle minerals. Earth and Planetary Science Letters 377, 311-323.
show that the silicate mantle may still contain an amount of nitrogen one to two orders of magnitude larger than the present atmospheric reservoir. Accordingly, the uniform δ15N value of −5 ‰ of Earth’s mantle may have been established before Archean. Because enstatite chondrites have δ15N of −45 to −15 ‰ (Grady et al., 1986Grady, M.M., Wright, I., Carr, L., Pillinger, C. (1986) Compositional differences in enstatite chondrites based on carbon and nitrogen stable isotope measurements. Geochimica et Cosmochimica Acta 50, 2799-2813.
), while carbonaceous chondrites have δ15N of +15 to +55 ‰ (Kerridge, 1985Kerridge, J.F. (1985) Carbon, hydrogen and nitrogen in carbonaceous chondrites: Abundances and isotopic compositions in bulk samples. Geochimica et Cosmochimica Acta 49, 1707-1714.
), the extremely negative δ15N values down to −25 ‰ and −40 ‰ observed in a few mantle diamonds are interpreted to be relicts of primordial nitrogen and are used to argue for an enstatite chondrite origin of Earth’s nitrogen (Javoy, 1997Javoy, M. (1997) The major volatile elements of the earth: Their origin, behavior, and fate. Geophysical Research Letters 24, 177-180.
; Palot et al., 2012Palot, M., Cartigny, P., Harris, J., Kaminsky, F., Stachel, T. (2012) Evidence for deep mantle convection and primordial heterogeneity from nitrogen and carbon stable isotopes in diamond. Earth and Planetary Science Letters 357, 179-193.
; Cartigny and Marty, 2013Cartigny, P., Marty, B. (2013) Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359-366.
). If this is correct, then one open question is how the Earth’s nitrogen isotopes evolved from the initial δ15N values of enstatite chondrites to the present-day mantle value of −5 ‰ and the positive values observed for OIBs.A significant fraction of nitrogen may have been segregated into the core during core-mantle separation (Roskosz et al., 2013
Roskosz, M., Bouhifd, M., Jephcoat, A., Marty, B., Mysen, B. (2013) Nitrogen solubility in molten metal and silicate at high pressure and temperature. Geochimica et Cosmochimica Acta 121, 15-28.
). However, so far the potential influence of core-mantle separation on Earth’s mantle nitrogen isotope evolution has never been investigated. Here we experimentally show that a significant nitrogen isotope fractionation occurs between liquid Fe-rich metal and coexisting silicate melt, and terrestrial core-mantle separation may have greatly enriched the silicate mantle in δ15N.top
Results and Discussion
Experimental results obtained at 1.5 to 7.0 GPa and 1600 to 1800 °C are given in Table 1. The nitrogen metal/silicate melt partition coefficient DNmetal/silicate ranges from 1 to 150. Figure 1 shows that regardless of pressure or temperature, DNmetal/silicate increases with increasing oxygen fugacity, as calculated from the composition of the coexisting quenched metal and silicate (Table S-1). The trend in Figure 1 can be rationalised if one assumes that under these very reducing conditions below the iron-wüstite buffer, nitrogen is mostly dissolved as N3- ion in the silicate melt (Kadik et al., 2011
Kadik, A., Kurovskaya, N., Ignat’ev, Y., Kononkova, N., Koltashev, V., Plotnichenko, V. (2011) Influence of oxygen fugacity on the solubility of nitrogen, carbon, and hydrogen in FeO-N2O-SiO2-Al2O3 melts in equilibrium with metallic iron at 1.5 GPa and 1400 °C. Geochemistry International 49, 429-438.
, 2013Kadik, A., Litvin, Y., Koltashev, V., Kryukova, E., Plotnichenko, V., Tsekhonya, T., Kononkova, N. (2013) Solution behavior of reduced N–H–O volatiles in FeO–Na2O–SiO2–Al2O3 melt equilibrated with molten Fe alloy at high pressure and temperature. Physics of the Earth and Planetary Interiors 214, 14-24.
; Li et al., 2015Li, Y., Huang, R., Wiedenbeck, M., Keppler, H. (2015) Nitrogen distribution between aqueous fluids and silicate melts. Earth and Planetary Science Letters 411, 218-228.
), while it dissolves as interstitial N atoms in the metal (Häglund et al., 1993Häglund, J., Fernández Guillermet, A., Grimvall, G., Körling, M. (1993) Theory of bonding in transition-metal carbides and nitrides. Physical Review B 48, 11685-11691.
). This would imply that the exchange reaction between silicate melt and metal may be written asEq. 1
2N3- (silicate) + 3/2 O2 = 2N (metal) + 3 O2- (silicate)
Kadik, A., Kurovskaya, N., Ignat’ev, Y., Kononkova, N., Koltashev, V., Plotnichenko, V. (2011) Influence of oxygen fugacity on the solubility of nitrogen, carbon, and hydrogen in FeO-N2O-SiO2-Al2O3 melts in equilibrium with metallic iron at 1.5 GPa and 1400 °C. Geochemistry International 49, 429-438.
, 2013Kadik, A., Litvin, Y., Koltashev, V., Kryukova, E., Plotnichenko, V., Tsekhonya, T., Kononkova, N. (2013) Solution behavior of reduced N–H–O volatiles in FeO–Na2O–SiO2–Al2O3 melt equilibrated with molten Fe alloy at high pressure and temperature. Physics of the Earth and Planetary Interiors 214, 14-24.
; Roskosz et al., 2013Roskosz, M., Bouhifd, M., Jephcoat, A., Marty, B., Mysen, B. (2013) Nitrogen solubility in molten metal and silicate at high pressure and temperature. Geochimica et Cosmochimica Acta 121, 15-28.
) and only a few hundred ppm nitrogen was present in our silicate melt, partial crystallisation during quench may not have caused exsolution of nitrogen. The observed systematic dependence of DNmetal/silicate on oxygen fugacity (Fig. 1) also precludes any significant exsolution of nitrogen during quench.
Figure 1 Nitrogen partition coefficients between liquid Fe-rich metal and silicate melt (DNmetal/silicate). The published DNmetal/silicate data (Kadik et al., 2011
Kadik, A., Kurovskaya, N., Ignat’ev, Y., Kononkova, N., Koltashev, V., Plotnichenko, V. (2011) Influence of oxygen fugacity on the solubility of nitrogen, carbon, and hydrogen in FeO-N2O-SiO2-Al2O3 melts in equilibrium with metallic iron at 1.5 GPa and 1400 °C. Geochemistry International 49, 429-438.
, 2013Kadik, A., Litvin, Y., Koltashev, V., Kryukova, E., Plotnichenko, V., Tsekhonya, T., Kononkova, N. (2013) Solution behavior of reduced N–H–O volatiles in FeO–Na2O–SiO2–Al2O3 melt equilibrated with molten Fe alloy at high pressure and temperature. Physics of the Earth and Planetary Interiors 214, 14-24.
; Roskosz et al., 2013Roskosz, M., Bouhifd, M., Jephcoat, A., Marty, B., Mysen, B. (2013) Nitrogen solubility in molten metal and silicate at high pressure and temperature. Geochimica et Cosmochimica Acta 121, 15-28.
) are plotted for comparison. DNmetal/silicate is a function of oxygen fugacity, regardless of pressure or temperature. The slope of the trend line is moderately lower than 0.75 expected based on Eq. 1, which could be due to a small fraction of nitrogen present as N2 in the silicate melt. Errors of DNmetal/silicate in this study are at 95 % confidence interval.The measured nitrogen metal/silicate melt isotope fractionation Δ15Nmetal-silicate ranges from −1.1 to −5.5 ‰ (Table 1; Fig. 2), with a mean value of −3.5 ± 1.7 ‰, indicating 15N-enrichment in the silicate melt relative to the coexisting liquid Fe-rich metal. Within the analytical uncertainties, there is no apparent temperature dependence of Δ15Nmetal-silicate over the interval of 1600-1800 °C, which might be partially due to the covariation of pressure and temperature. The consistent Δ15Nmetal-silicate values from piston cylinder and multi-anvil experiments (Table 1) rule out the possibility that a significant change in Δ15Nmetal-silicate occurred during quench of the multi-anvil experiments. The nearly constant Δ15Nmetal-silicate values, independent on the run duration (30 to 120 mins; Table 1), also demonstrate that isotopic equilibrium was reached during the run and any kinetic fractionation should be within the analytical error.
Table 1 Experimental results on nitrogen partitioning and isotope fractionation between liquid Fe-rich metal and silicate melt.
| Exp. ID | P | T | Run duration | alogƒO2(ΔIW) | N con. in metal | N con. in silicate | δ15N of metal | δ15N of silicate | DNmetal/silicate | Δ15Nmetal−silicate | ||||||||||||||
| GPa | °C | mins | mol/g | mol/g | ||||||||||||||||||||
| Piston Cylinder Experiments | ||||||||||||||||||||||||
| Y-1 | 2.0 | 1600 | 120 | -0.84 | ± | 0.07 | 3.7E-04 | ± | 1.3E-05 | 2.2E-05 | ± | 7.9E-07 | 9.2 | ± | 1.6 | 13.3 | ± | 1.8 | 17 | ± | 1 | -4.1 | ± | 2.4 |
| A-659 | 1.5 | 1600 | 90 | -1.2 | ± | 0.17 | 4.9E-04 | ± | 1.8E-05 | 6.2E-05 | ± | 2.3E-06 | -1.1 | ± | 2.0 | 2.6 | ± | 1.6 | 8 | ± | 0.4 | -3.8 | ± | 2.5 |
| A-666 | 2.5 | 1700 | 30 | n.d. | 2.6E-06 | ± | 9.4E-08 | 3.4E-06 | ± | 1.3E-07 | -3.3 | ± | 2.4 | -2.2 | ± | 1.4 | 1 | ± | 0.04 | -1.1 | ± | 2.8 | ||
| Multi-Anvil Experiments | ||||||||||||||||||||||||
| YS-5 | 5.0 | 1700 | 90 | -0.66 | ± | 0.11 | 2.4E-03 | ± | 8.7E-05 | 1.6E-05 | ± | 5.8E-07 | 1.7 | ± | 1.5 | 7.2 | ± | 1.7 | 149 | ± | 8 | -5.5 | ± | 2.3 |
| YS-1 | 7.0 | 1700 | 90 | n.d. | 7.2E-04 | ± | 2.6E-05 | 3.6E-05 | ± | 1.3E-06 | -0.6 | ± | 1.9 | 1.4 | ± | 1.7 | 20 | ± | 1 | -2.0 | ± | 2.5 | ||
| YS-2 | 7.0 | 1800 | 90 | -0.83 | ± | 0.11 | 1.0E-03 | ± | 3.8E-05 | 2.5E-05 | ± | 6.8E-07 | -2.0 | ± | 2.1 | 2.6 | ± | 1.5 | 42 | ± | 2 | -4.6 | ± | 2.6 |
a: The logƒO2(ΔIW) was calcualted using this equilibrium: FeO (silicate melt) = Fe (liquid metal) + 1/2O2, from which the ƒO2 of the experiment relative to ƒO2 of the iron-wüstite buffer (IW) can be defined as: ΔIW = 2 log (XFeOγFeO/XFeγFe), XFeO and XFe are the mole fractions of FeO in silicate melt and Fe in liquid metal, respectively;
γFeO and γFe are the activity coefficients of FeO in silicate melt and Fe in liquid metal, respectively.
Calculation of logƒO2(ΔIW) was performed assuming γFeO = 1.2 in the silicate melt (O'Neill and Eggins, 2002
O'Neill, H.S.C., Eggins, S.M. (2002) The effect of melt composition on trace element partitioning: an experimental investigation of the activity coefficients of FeO, NiO, CoO, MoO2 and MoO3 in silicate melts. Chemical Geology 186, 151-181.
) and ideal solution of liquid Fe-C-Pt-N metal (γFe = 1).
For major element compositions of silicate melt and liquid metal, see Supplementary Table 1.
n.d.: not determined.
Errors for DNmetal/silicate and Δ15Nmetal-silicate are at 95 % confidence interval.

Figure 2 Nitrogen isotope fractionation between liquid Fe-rich metal and silicate melt (Δ15Nmetal-silicate). Temperature has a very limited effect on Δ15Nmetal-silicate in the P-T range studied. Errors of Δ15Nmetal-silicate are at 95 % confidence interval.
top
Implications
The DNmetal/silicate and Δ15Nmetal-silicate obtained here have important implications for the origin and evolution of Earth’s nitrogen. The DNmetal/silicate values of 1 to 150 demonstrate that a large fraction of nitrogen in the magma ocean may have segregated into the core, while the Δ15Nmetal-silicate values of −1.1 to −5.5 ‰ imply that terrestrial core-mantle separation may have enriched the silicate mantle in 15N. There are two endmember models for terrestrial core formation. The first one is the single stage model or equilibrium model, in which chemical equilibrium between the core and the mantle is thought to be achieved at certain P-T conditions at the base of the magma ocean (Li and Agee, 1996
Li, J., Agee, C.B. (1996) Geochemistry of mantle-core differentiation at high pressure. Nature 381, 686-689.
; Righter, 2011Righter, K. (2011) Prediction of metal–silicate partition coefficients for siderophile elements: An update and assessment of PT conditions for metal–silicate equilibrium during accretion of the Earth. Earth and Planetary Science Letters 304, 158-167.
). This equilibrium model leads to the below mass balance for the segregation of nitrogen in the coreEq. 2
δ15Nmantle×XNmantle + δ15Ncore × XNcore = δ15NBE
Eq. 3
DNmetal/silicate × CNmantle = CNcore
Eq. 4
δ15Ncore - δ15Nmantle = Δ15Nmetal-silicate
Marty, B. (2012) The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth and Planetary Science Letters 313-314, 56-66.
is used, then the CNcore would be 4-16 ppm.The second endmember mode is the continuous core formation model (Wood et al., 2006
Wood, B.J., Walter, M.J., Wade, J. (2006) Accretion of the Earth and segregation of its core. Nature 441, 825-832.
, 2013Wood, B.J., Li, J., Shahar, A. (2013) Carbon in the core: its influence on the properties of core and mantle. Reviews in Mineralogy and Geochememistry 75, 231-250.
). In this model, Earth accretion and the delivery of core-forming metal occur in small steps of 1 % mass with constant metal/silicate ratio. In each step the metal equilibrates with the silicate magma ocean, and it remains chemically isolated once it segregates in the core. The fractionation of light element isotopes in this model can best be described by the Rayleigh distillation model (Wood et al., 2013Wood, B.J., Li, J., Shahar, A. (2013) Carbon in the core: its influence on the properties of core and mantle. Reviews in Mineralogy and Geochememistry 75, 231-250.
; Horita and Polyakov, 2015Horita, J., Polyakov, V.B. (2015) Carbon-bearing iron phases and the carbon isotope composition of the deep Earth. Proceedings of the National Academy of Sciences 112, 31-36.
)Eq. 5
δ15Nmantle = δ15NBE + Δ15Nmetal-silicate × lnƒNmantle
Eq. 6
ƒNmantle = (CNmantle × Mmantle)/(CNBE × MBE)
Eq. 7
CNmantle = CNBE × (Mmantle/MBE)(DNmetal-silicate-1)
Grady, M.M., Wright, I., Carr, L., Pillinger, C. (1986) Compositional differences in enstatite chondrites based on carbon and nitrogen stable isotope measurements. Geochimica et Cosmochimica Acta 50, 2799-2813.
), this model implies that core-mantle separation may cause a significant increase in δ15Nmantle and may reproduce the δ15Nmantle range, if DNmetal/silicate is between 5 and 20 (Fig. 3). Using the CNmantle of 0.8 ppm by Marty (2012)Marty, B. (2012) The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth and Planetary Science Letters 313-314, 56-66.
and a DNmetal/silicate of 5-15, the calculated CNBE is 4-220 ppm (excluding surface nitrogen) and the CNcore is 10-660 ppm. However, if a DNmetal/silicate of 15-20 is used, the calculated CNBE is 220-1600 ppm and the CNcore is 660-5000 ppm. The high end of these CNBE values may be higher than the nitrogen concentration in any known chondrites (Krot et al., 2014Krot, A.N., Keil, K., Scott, E.R.D., Goodrich, C.A., Weisberg, M.K. (2014) Classification of meteorites and their genetic relationships. Treatise on Geochemistry 1, 1-63.
), which thus indicates that a hybrid Rayleigh-equilibrium model may be more realistic for the segregation of nitrogen in Earth’s core if DNmetal/silicate is 15-20. Such a hybrid model (Rubie et al., 2015Rubie, D.C., Jacobson, S.A., Morbidelli, A., O’Brien, D.P., Young, E.D., de Vries, J., Nimmo, F., Palme, H., Frost, D.J. (2015) Accretion and differentiation of the terrestrial planets with implications for the compositions of early-formed Solar System bodies and accretion of water. Icarus 248, 89-108.
) assumes that during the early stages of accretion, the impacting objects are relatively small and their metal phase sequentially extracts nitrogen from the magma ocean according to a Rayleigh model; however, towards the end of accretion, large, differentiated planetesimals collide with the growing Earth. The cores of these large objects are in bulk equilibrium with their mantles, but due to their large size, they do not exchange nitrogen with the silicate magma ocean anymore.
Figure 3 The modelled δ15N value of the silicate mantle as a function of DNmetal/silicate, just after complete core-mantle separation, using the Rayleigh distillation model (Eq. 5) and the relationship between ƒNmantle and DNmetal/silicate (Eqs. 6 and 7). Three different δ15NBE values are used: the lowest value (−45 ‰) is the lowest value observed so far for Earth’s mantle and enstatite chondrites (Grady et al., 1986
Grady, M.M., Wright, I., Carr, L., Pillinger, C. (1986) Compositional differences in enstatite chondrites based on carbon and nitrogen stable isotope measurements. Geochimica et Cosmochimica Acta 50, 2799-2813.
; Palot et al., 2012Palot, M., Cartigny, P., Harris, J., Kaminsky, F., Stachel, T. (2012) Evidence for deep mantle convection and primordial heterogeneity from nitrogen and carbon stable isotopes in diamond. Earth and Planetary Science Letters 357, 179-193.
); the values of −25 ‰ and −15 ‰ correspond to the average and highest δ15N values of enstatite chondrites (Grady et al., 1986Grady, M.M., Wright, I., Carr, L., Pillinger, C. (1986) Compositional differences in enstatite chondrites based on carbon and nitrogen stable isotope measurements. Geochimica et Cosmochimica Acta 50, 2799-2813.
), respectively. Two different values of −2 ‰ and −5 ‰ are used for the dashed lines and solid lines, respectively. The gray box represents the main range of δ15N values observed for the silicate mantle (Marty and Dauphas, 2003Marty, B., Dauphas, N. (2003) The nitrogen record of crust-mantle interaction and mantle convection from Archean to Present. Earth and Planetary Science Letters 206, 397-410.
; Palot et al., 2012Palot, M., Cartigny, P., Harris, J., Kaminsky, F., Stachel, T. (2012) Evidence for deep mantle convection and primordial heterogeneity from nitrogen and carbon stable isotopes in diamond. Earth and Planetary Science Letters 357, 179-193.
; Cartigny and Marty, 2013Cartigny, P., Marty, B. (2013) Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359-366.
), which may be produced from the average nitrogen isotopic composition of enstatite chondrites by core-mantle separation with DNmetal/silicate between 5 and 20.In a hybrid Rayleigh-equilibrium model, the δ15Nmantle after complete core-mantle separation should be between the δ15Nmantle constrained by the Rayleigh model and the δ15Nmantle constrained by the equilibrium model. For example, in a 90 % Rayleigh and 10 % equilibrium model, the resulting δ15Nmantle would be ‒17 ‰ at a DNmetal/silicate of 15, a Δ15Nmetal-silicate of ‒5 ‰, and the average enstatite chondrite δ15N value of ‒25 ‰. The CNBE required to achieve the CNmantle of 0.8 ppm by Marty (2012)
Marty, B. (2012) The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth and Planetary Science Letters 313-314, 56-66.
would be about 40 ppm. According to Rubie et al. (2015)Rubie, D.C., Jacobson, S.A., Morbidelli, A., O’Brien, D.P., Young, E.D., de Vries, J., Nimmo, F., Palme, H., Frost, D.J. (2015) Accretion and differentiation of the terrestrial planets with implications for the compositions of early-formed Solar System bodies and accretion of water. Icarus 248, 89-108.
, 80-100 % of the core-forming metal may have equilibrated with the silicate magma ocean according to the Rayleigh model. However, it should be noted that the resulting δ15Nmantle of ‒17 ‰ in the hybrid model above is still significantly lower than the present-day mantle δ15N of −5 ‰, which indicates a much reduced efficiency of the hybrid Rayleigh-equilibrium model in increasing δ15Nmantle compared to the pure Rayleigh model.Our above calculations demonstrate that: (1) the bulk Earth δ15N value has to be significantly negative to produce the present-day mantle δ15N of −5 ‰; (2) core-mantle separation alone may be sufficient to cause the present-day mantle δ15N of −5 ‰ even if the Earth accreted only from the enstatite chondrites, if the Rayleigh model is used for describing the segregation of nitrogen in Earth’s core; (3) as has been suggested previously (Javoy, 1997
Javoy, M. (1997) The major volatile elements of the earth: Their origin, behavior, and fate. Geophysical Research Letters 24, 177-180.
), a small fraction of carbonaceous chondrites with δ15N of +15 to +55 ‰ may have to be added to the Earth to achieve the present-day mantle δ15N of −5 ‰, if the Earth accreted mainly from enstatite chondrites and if the equilibrium or the hybrid Rayleigh-equilibrium model is used for describing the segregation of nitrogen in Earth’s core. These results support previous models (Javoy, 1997Javoy, M. (1997) The major volatile elements of the earth: Their origin, behavior, and fate. Geophysical Research Letters 24, 177-180.
; Palot et al., 2012Palot, M., Cartigny, P., Harris, J., Kaminsky, F., Stachel, T. (2012) Evidence for deep mantle convection and primordial heterogeneity from nitrogen and carbon stable isotopes in diamond. Earth and Planetary Science Letters 357, 179-193.
; Cartigny and Marty, 2013Cartigny, P., Marty, B. (2013) Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359-366.
) that assumed enstatite chondrites to be the main source of Earth’s nitrogen. These results are also consistent with several other isotopic systems that point towards enstatite chondrites as a main source of the material from which the Earth formed (Javoy, 1997Javoy, M. (1997) The major volatile elements of the earth: Their origin, behavior, and fate. Geophysical Research Letters 24, 177-180.
; Trinquier et al., 2007Trinquier, A., Birck, J., Allègre, C.J. (2007) Widespread 54Cr heterogeneity in the inner solar system. The Astrophysical Journal 655, 1179.
; Regelous et al., 2008Regelous, M., Elliott, T., Coath, C.D. (2008) Nickel isotope heterogeneity in the early Solar System. Earth and Planetary Science Letters 272, 330-338.
; Javoy et al., 2010Javoy, M, Kaminski, E., Guyot, F., Andrault, D., Sanloup, C., Moreira, M., Labrosse, S., Jambon, A., Agrinier, P., Davaille, A., Jaupart, C. (2010) The chemical composition of the Earth: Enstatite chondrite models. Earth and Planetary Science Letters 293, 259-268.
).The Earth’s deep mantle below 250 km is reducing with oxygen fugacity lower than the iron-wüstite buffer (Frost and McCammon, 2008
Frost, D.J., McCammon, C.A. (2008) The redox state of Earth's mantle. Annual Review of Earth and Planetary Sciences 36, 389-420.
). Recent experimental studies (Frost et al., 2004Frost, D. Liebske, C., Langenhorst, F., McCammon, C. (2004) Experimental evidence for the existence of iron-rich metal in the Earth's lower mantle. Nature 428, 409-412.
; Rohrbach and Schmidt, 2011Rohrbach, A., Schmidt, M.W. (2011) Redox freezing and melting in the Earth's deep mantle resulting from carbon-iron redox coupling. Nature 472, 209-212.
) suggest that about 1 wt. % Fe-rich metal may be stable in the mantle below this depth, because of the incorporation of significant Fe3+ in majoritic garnet and bridgmanite according to the disproportion reaction 3Fe2+ = 2Fe3+ + Fe0, which produces metallic Fe. The moderately siderophile nature of nitrogen together with the relatively low nitrogen solubility in mantle minerals (Li et al., 2013Li, Y., Wiedenbeck, M., Shcheka, S., Keppler, H. (2013) Nitrogen solubility in upper mantle minerals. Earth and Planetary Science Letters 377, 311-323.
) implies that 1 wt. % Fe-rich metal may store more than 99 % of the nitrogen in the deep mantle. If one assumes negligible nitrogen isotope fractionation between silicate minerals and silicate melt at temperatures corresponding to the deep mantle, the silicate minerals should be enriched in δ15N by +1 to +5.5 ‰ relative to the Fe-rich metal. Partial melting of the deep mantle with δ15N of −5 ‰, in the presence of 0.5 to 1.5 wt. % Fe-rich metal (Rohrbach et al., 2014Rohrbach, A., Ghosh, S., Schmidt, M.W., Wijbrans, C.H., Klemme, S. (2014) The stability of Fe–Ni carbides in the Earth's mantle: Evidence for a low Fe–Ni–C melt fraction in the deep mantle. Earth and Planetary Science Letters 388, 211-221.
), may therefore generate OIBs with very slightly positive δ15N values; however, generating OIBs with δ15N up to +8 ‰ may need the source region δ15N > +0 ‰.top
Acknowledgements
We thank Hubert Schulze for sample preparation. Y.L. acknowledges support from the Elite Network Bavaria (ENB) program. Constructive reviews by three anonymous reviewers and journal editor Bruce Watson helped to improve this paper.
Editor: Bruce Watson
top
References
Cartigny, P., Marty, B. (2013) Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359-366.
 Show in context
Show in context
The present-day upper mantle δ15N, inferred from fibrous diamonds and mid-ocean ridge basalts (MORBs) is −12 to 0 ‰ and converges towards a globally uniform value of −5 ‰ (Marty and Dauphas, 2003; Cartigny and Marty, 2013).
View in article
The δ15N value of the organic matter and metasediments is overall positive, and the δ15N value of Earth’s surface (crust + atmosphere) is approximately +2 ‰ (Cartigny and Marty, 2013; Thomazo and Papineau, 2013).
View in article
Because enstatite chondrites have δ15N of −45 to −15 ‰ (Grady et al., 1986), while carbonaceous chondrites have δ15N of +15 to +55 ‰ (Kerridge, 1985), the extremely negative δ15N values down to −25 ‰ and −40 ‰ observed in a few mantle diamonds are interpreted to be relicts of primordial nitrogen and are used to argue for an enstatite chondrite origin of Earth’s nitrogen (Javoy, 1997; Palot et al., 2012; Cartigny and Marty, 2013).
View in article
Figure 3 [...] The gray box represents the main range of &delta:15N values observed for the silicate mantle (Marty and Dauphas, 2003; Palot et al., 2012; Cartigny and Marty, 2013), which may be produced from the average nitrogen isotopic composition of enstatite chondrites by core-mantle separation with DNmetal/silicate between 5 and 20.
View in article
These results support previous models (Javoy, 1997; Palot et al., 2012; Cartigny and Marty, 2013) that assumed enstatite chondrites to be the main source of Earth’s nitrogen.
View in article
Cartigny, P., Farquhar, J., Thomassot, E., Harris, J., Wing, B., Masterson, A., McKeegan, K., Stachel, T. (2009) A mantle origin for Paleoarchean peridotitic diamonds from the Panda kimberlite, Slave Craton: evidence from 13C-, 15N-and 33,34S-stable isotope systematics. Lithos 112, 852-864.
 Show in context
Show in context
Nevertheless, most diamond populations with Archean ages define a δ15N range of −12 to +5 ‰, with a mode around −5 ‰ (Cartigny et al., 2009), indicating no obvious secular change in mantle δ15N and thus limited nitrogen recycling compared to the nitrogen abundance in the mantle.
View in article
Dauphas, N., Marty, B. (1999) Heavy nitrogen in carbonatites of the Kola Peninsula: A possible signature of the deep mantle. Science 286, 2488-2490.
 Show in context
Show in context
The oceanic island basalts (OIBs), thought to be derived from the lower mantle (e.g., Shen et al., 1998; Stuart et al., 2003), have δ15N of −2 to +8 ‰, with a mean value of about +3 ‰ (Dauphas and Marty, 1999; Marty and Dauphas, 2003).
View in article
Therefore the positive δ15N values of OIBs were interpreted to be results of addition of recycled sediments to the OIB source region (Dauphas and Marty, 1999; Marty and Dauphas, 2003).
View in article
Frost, D.J., McCammon, C.A. (2008) The redox state of Earth's mantle. Annual Review of Earth and Planetary Sciences 36, 389-420.
 Show in context
Show in context
The Earth’s deep mantle below 250 km is reducing with oxygen fugacity lower than the iron-wüstite buffer (Frost and McCammon, 2008).
View in article
Frost, D. Liebske, C., Langenhorst, F., McCammon, C. (2004) Experimental evidence for the existence of iron-rich metal in the Earth's lower mantle. Nature 428, 409-412.
 Show in context
Show in context
Recent experimental studies (Frost et al., 2004; Rohrbach and Schmidt, 2011) suggest that about 1 wt. % Fe-rich metal may be stable in the mantle below this depth, because of the incorporation of significant Fe3+ in majoritic garnet and bridgmanite according to the disproportion reaction 3Fe2+ = 2Fe3+ + Fe0, which produces metallic Fe.
View in article
Grady, M.M., Wright, I., Carr, L., Pillinger, C. (1986) Compositional differences in enstatite chondrites based on carbon and nitrogen stable isotope measurements. Geochimica et Cosmochimica Acta 50, 2799-2813.
 Show in context
Show in context
Because enstatite chondrites have δ15N of −45 to −15 ‰ (Grady et al., 1986), while carbonaceous chondrites have δ15N of +15 to +55 ‰ (Kerridge, 1985), the extremely negative δ15N values down to −25 ‰ and −40 ‰ observed in a few mantle diamonds are interpreted to be relicts of primordial nitrogen and are used to argue for an enstatite chondrite origin of Earth’s nitrogen (Javoy, 1997; Palot et al., 2012; Cartigny and Marty, 2013).
View in article
For different δ15NBE values that represent the δ15N range of enstatite chondrites (Grady et al., 1986), this model implies that core-mantle separation may cause a significant increase in δ15Nmantle and may reproduce the δ15Nmantle range, if DNmetal/silicate is between 5 and 20 (Fig. 3).
View in article
Figure 3 [...] Three different δ15NBE values are used: the lowest value (−45 ‰) is the lowest value observed so far for Earth’s mantle and enstatite chondrites (Grady et al., 1986; Palot et al., 2012); the values of −25 ‰ and −15 ‰ correspond to the average and highest δ15N values of enstatite chondrites (Grady et al., 1986), respectively.
View in article
Häglund, J., Fernández Guillermet, A., Grimvall, G., Körling, M. (1993) Theory of bonding in transition-metal carbides and nitrides. Physical Review B 48, 11685-11691.
 Show in context
Show in context
The trend in Figure 1 can be rationalised if one assumes that under these very reducing conditions below the iron-wüstite buffer, nitrogen is mostly dissolved as N3- ion in the silicate melt (Kadik et al., 2011, 2013; Li et al., 2015), while it dissolves as interstitial N atoms in the metal (Häglund et al., 1993).
View in article
Horita, J., Polyakov, V.B. (2015) Carbon-bearing iron phases and the carbon isotope composition of the deep Earth. Proceedings of the National Academy of Sciences 112, 31-36.
 Show in context
Show in context
The fractionation of light element isotopes in this model can best be described by the Rayleigh distillation model (Wood et al., 2013; Horita and Polyakov, 2015): δ15Nmantle = δ15NBE + Δ15Nmetal-silicate × lnƒNmantle where ƒNmantle is the remaining fraction of nitrogen in the silicate mantle just after complete core-mantle separation.
View in article
Javoy, M. (1997) The major volatile elements of the earth: Their origin, behavior, and fate. Geophysical Research Letters 24, 177-180.
 Show in context
Show in context
Nitrogen isotopes may constrain the origin of terrestrial volatiles (Javoy, 1997; Marty, 2012), as well as the evolution and interaction of different terrestrial reservoirs (Marty and Dauphas, 2003).
View in article
Because enstatite chondrites have δ15N of −45 to −15 ‰ (Grady et al., 1986), while carbonaceous chondrites have δ15N of +15 to +55 ‰ (Kerridge, 1985), the extremely negative δ15N values down to −25 ‰ and −40 ‰ observed in a few mantle diamonds are interpreted to be relicts of primordial nitrogen and are used to argue for an enstatite chondrite origin of Earth’s nitrogen (Javoy, 1997; Palot et al., 2012; Cartigny and Marty, 2013).
View in article
Our above calculations demonstrate that: (1) the bulk Earth δ15N value has to be significantly negative to produce the present-day mantle δ15N of −5 ‰; (2) core-mantle separation alone may be sufficient to cause the present-day mantle δ15N of −5 ‰ even if the Earth accreted only from the enstatite chondrites, if the Rayleigh model is used for describing the segregation of nitrogen in Earth’s core; (3) as has been suggested previously (Javoy, 1997), a small fraction of carbonaceous chondrites with δ15N of +15 to +55 ‰ may have to be added to the Earth to achieve the present-day mantle δ15N of −5 ‰, if the Earth accreted mainly from enstatite chondrites and if the equilibrium or the hybrid Rayleigh-equilibrium model is used for describing the segregation of nitrogen in Earth’s core.
View in article
These results support previous models (Javoy, 1997; Palot et al., 2012; Cartigny and Marty, 2013) that assumed enstatite chondrites to be the main source of Earth’s nitrogen.
View in article
These results are also consistent with several other isotopic systems that point towards enstatite chondrites as a main source of the material from which the Earth formed (Javoy, 1997; Trinquier et al., 2007; Regelous et al., 2008; Javoy et al., 2010).
View in article
Javoy, M, Kaminski, E., Guyot, F., Andrault, D., Sanloup, C., Moreira, M., Labrosse, S., Jambon, A., Agrinier, P., Davaille, A., Jaupart, C. (2010) The chemical composition of the Earth: Enstatite chondrite models. Earth and Planetary Science Letters 293, 259-268.
 Show in context
Show in context
These results are also consistent with several other isotopic systems that point towards enstatite chondrites as a main source of the material from which the Earth formed (Javoy, 1997; Trinquier et al., 2007; Regelous et al., 2008; Javoy et al., 2010).
View in article
Kadik, A., Kurovskaya, N., Ignat’ev, Y., Kononkova, N., Koltashev, V., Plotnichenko, V. (2011) Influence of oxygen fugacity on the solubility of nitrogen, carbon, and hydrogen in FeO-N2O-SiO2-Al2O3 melts in equilibrium with metallic iron at 1.5 GPa and 1400 °C. Geochemistry International 49, 429-438.
 Show in context
Show in context
The trend in Figure 1 can be rationalised if one assumes that under these very reducing conditions below the iron-wüstite buffer, nitrogen is mostly dissolved as N3- ion in the silicate melt (Kadik et al., 2011, 2013; Li et al., 2015), while it dissolves as interstitial N atoms in the metal (Häglund et al., 1993).
View in article
However, considering that nitrogen solubility in reduced silicate melt ranges up to a few wt. % (Kadik et al., 2011, 2013; Roskosz et al., 2013) and only a few hundred ppm nitrogen was present in our silicate melt, partial crystallisation during quench may not have caused exsolution of nitrogen.
View in article
Figure 1 [...] The published DNmetal/silicate data (Kadik et al., 2011, 2013; Roskosz et al., 2013) are plotted for comparison.
View in article
Kadik, A., Litvin, Y., Koltashev, V., Kryukova, E., Plotnichenko, V., Tsekhonya, T., Kononkova, N. (2013) Solution behavior of reduced N–H–O volatiles in FeO–Na2O–SiO2–Al2O3 melt equilibrated with molten Fe alloy at high pressure and temperature. Physics of the Earth and Planetary Interiors 214, 14-24.
 Show in context
Show in context
The trend in Figure 1 can be rationalised if one assumes that under these very reducing conditions below the iron-wüstite buffer, nitrogen is mostly dissolved as N3- ion in the silicate melt (Kadik et al., 2011, 2013; Li et al., 2015), while it dissolves as interstitial N atoms in the metal (Häglund et al., 1993).
View in article
However, considering that nitrogen solubility in reduced silicate melt ranges up to a few wt. % (Kadik et al., 2011, 2013; Roskosz et al., 2013) and only a few hundred ppm nitrogen was present in our silicate melt, partial crystallisation during quench may not have caused exsolution of nitrogen.
View in article
Figure 1 [...] The published DNmetal/silicate data (Kadik et al., 2011, 2013; Roskosz et al., 2013) are plotted for comparison.
View in article
Kerridge, J.F. (1985) Carbon, hydrogen and nitrogen in carbonaceous chondrites: Abundances and isotopic compositions in bulk samples. Geochimica et Cosmochimica Acta 49, 1707-1714.
 Show in context
Show in context
Because enstatite chondrites have δ15N of −45 to −15 ‰ (Grady et al., 1986), while carbonaceous chondrites have δ15N of +15 to +55 ‰ (Kerridge, 1985), the extremely negative δ15N values down to −25 ‰ and −40 ‰ observed in a few mantle diamonds are interpreted to be relicts of primordial nitrogen and are used to argue for an enstatite chondrite origin of Earth’s nitrogen (Javoy, 1997; Palot et al., 2012; Cartigny and Marty, 2013).
View in article
Krot, A.N., Keil, K., Scott, E.R.D., Goodrich, C.A., Weisberg, M.K. (2014) Classification of meteorites and their genetic relationships. Treatise on Geochemistry 1, 1-63.
 Show in context
Show in context
The high end of these CNBE values may be higher than the nitrogen concentration in any known chondrites (Krot et al., 2014), which thus indicates that a hybrid Rayleigh-equilibrium model may be more realistic for the segregation of nitrogen in Earth’s core if DNmetal/silicate is 15-20.
View in article
Li, J., Agee, C.B. (1996) Geochemistry of mantle-core differentiation at high pressure. Nature 381, 686-689.
 Show in context
Show in context
The first one is the single stage model or equilibrium model, in which chemical equilibrium between the core and the mantle is thought to be achieved at certain P-T conditions at the base of the magma ocean (Li and Agee, 1996; Righter, 2011).
View in article
Li, Y., Wiedenbeck, M., Shcheka, S., Keppler, H. (2013) Nitrogen solubility in upper mantle minerals. Earth and Planetary Science Letters 377, 311-323.
 Show in context
Show in context
Li et al. (2013) show that the silicate mantle may still contain an amount of nitrogen one to two orders of magnitude larger than the present atmospheric reservoir.
View in article
The moderately siderophile nature of nitrogen together with the relatively low nitrogen solubility in mantle minerals (Li et al., 2013) implies that 1 wt. % Fe-rich metal may store more than 99 % of the nitrogen in the deep mantle.
View in article
Li, Y., Huang, R., Wiedenbeck, M., Keppler, H. (2015) Nitrogen distribution between aqueous fluids and silicate melts. Earth and Planetary Science Letters 411, 218-228.
 Show in context
Show in context
The trend in Figure 1 can be rationalised if one assumes that under these very reducing conditions below the iron-wüstite buffer, nitrogen is mostly dissolved as N3- ion in the silicate melt (Kadik et al., 2011, 2013; Li et al., 2015), while it dissolves as interstitial N atoms in the metal (Häglund et al., 1993).
View in article
Marty, B. (2012) The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth and Planetary Science Letters 313-314, 56-66.
 Show in context
Show in context
Nitrogen isotopes may constrain the origin of terrestrial volatiles (Javoy, 1997; Marty, 2012), as well as the evolution and interaction of different terrestrial reservoirs (Marty and Dauphas, 2003).
View in article
In this case, if the CNmantle of 0.8 ppm constrained by Marty (2012) is used, then the CNcore would be 4-16 ppm.
View in article
Using the CNmantle of 0.8 ppm by Marty (2012) and a DNmetal/silicate of 5-15, the calculated CNBE is 4-220 ppm (excluding surface nitrogen) and the CNcore is 10-660 ppm.
View in article
The CNBE required to achieve the CNmantle of 0.8 ppm by Marty (2012) would be about 40 ppm.
View in article
Marty, B., Dauphas, N. (2003) The nitrogen record of crust-mantle interaction and mantle convection from Archean to Present. Earth and Planetary Science Letters 206, 397-410.
 Show in context
Show in context
Nitrogen isotopes may constrain the origin of terrestrial volatiles (Javoy, 1997; Marty, 2012), as well as the evolution and interaction of different terrestrial reservoirs (Marty and Dauphas, 2003).
View in article
The present-day upper mantle δ15N, inferred from fibrous diamonds and mid-ocean ridge basalts (MORBs) is −12 to 0 ‰ and converges towards a globally uniform value of −5 ‰ (Marty and Dauphas, 2003; Cartigny and Marty, 2013).
View in article
The oceanic island basalts (OIBs), thought to be derived from the lower mantle (e.g., Shen et al., 1998; Stuart et al., 2003), have δ15N of −2 to +8 ‰, with a mean value of about +3 ‰ (Dauphas and Marty, 1999; Marty and Dauphas, 2003).
View in article
Therefore the positive δ15N values of OIBs were interpreted to be results of addition of recycled sediments to the OIB source region (Dauphas and Marty, 1999; Marty and Dauphas, 2003).
View in article
Figure 3 [...] The gray box represents the main range of &delta:15N values observed for the silicate mantle (Marty and Dauphas, 2003; Palot et al., 2012; Cartigny and Marty, 2013), which may be produced from the average nitrogen isotopic composition of enstatite chondrites by core-mantle separation with DNmetal/silicate between 5 and 20.
View in article
O'Neill, H.S.C., Eggins, S.M. (2002) The effect of melt composition on trace element partitioning: an experimental investigation of the activity coefficients of FeO, NiO, CoO, MoO2 and MoO3 in silicate melts. Chemical Geology 186, 151-181.
 Show in context
Show in context
Table 1 [...] Calculation of logƒO2(ΔIW) was performed assuming γFeO = 1.2 in the silicate melt (O'Neill and Eggins, 2002) and ideal solution of liquid Fe-C-Pt-N metal (γFe = 1).
View in article
Palot, M., Cartigny, P., Harris, J., Kaminsky, F., Stachel, T. (2012) Evidence for deep mantle convection and primordial heterogeneity from nitrogen and carbon stable isotopes in diamond. Earth and Planetary Science Letters 357, 179-193.
 Show in context
Show in context
However, diamonds derived from the mantle transition zone and lower mantle show similar δ15N values to the upper mantle (Palot et al., 2012).
View in article
Because enstatite chondrites have δ15N of −45 to −15 ‰ (Grady et al., 1986), while carbonaceous chondrites have δ15N of +15 to +55 ‰ (Kerridge, 1985), the extremely negative δ15N values down to −25 ‰ and −40 ‰ observed in a few mantle diamonds are interpreted to be relicts of primordial nitrogen and are used to argue for an enstatite chondrite origin of Earth’s nitrogen (Javoy, 1997; Palot et al., 2012; Cartigny and Marty, 2013).
View in article
Figure 3 [...] Three different δ15NBE values are used: the lowest value (−45 ‰) is the lowest value observed so far for Earth’s mantle and enstatite chondrites (Grady et al., 1986; Palot et al., 2012); the values of −25 ‰ and −15 ‰ correspond to the average and highest δ15N values of enstatite chondrites (Grady et al., 1986), respectively.
View in article
Figure 3 [...] The gray box represents the main range of &delta:15N values observed for the silicate mantle (Marty and Dauphas, 2003; Palot et al., 2012; Cartigny and Marty, 2013), which may be produced from the average nitrogen isotopic composition of enstatite chondrites by core-mantle separation with DNmetal/silicate between 5 and 20.
View in article
These results support previous models (Javoy, 1997; Palot et al., 2012; Cartigny and Marty, 2013) that assumed enstatite chondrites to be the main source of Earth’s nitrogen.
View in article
Regelous, M., Elliott, T., Coath, C.D. (2008) Nickel isotope heterogeneity in the early Solar System. Earth and Planetary Science Letters 272, 330-338.
 Show in context
Show in context
These results are also consistent with several other isotopic systems that point towards enstatite chondrites as a main source of the material from which the Earth formed (Javoy, 1997; Trinquier et al., 2007; Regelous et al., 2008; Javoy et al., 2010).
View in article
Righter, K. (2011) Prediction of metal–silicate partition coefficients for siderophile elements: An update and assessment of PT conditions for metal–silicate equilibrium during accretion of the Earth. Earth and Planetary Science Letters 304, 158-167.
 Show in context
Show in context
The first one is the single stage model or equilibrium model, in which chemical equilibrium between the core and the mantle is thought to be achieved at certain P-T conditions at the base of the magma ocean (Li and Agee, 1996; Righter, 2011).
View in article
Rohrbach, A., Schmidt, M.W. (2011) Redox freezing and melting in the Earth's deep mantle resulting from carbon-iron redox coupling. Nature 472, 209-212.
 Show in context
Show in context
Recent experimental studies (Frost et al., 2004; Rohrbach and Schmidt, 2011) suggest that about 1 wt. % Fe-rich metal may be stable in the mantle below this depth, because of the incorporation of significant Fe3+ in majoritic garnet and bridgmanite according to the disproportion reaction 3Fe2+ = 2Fe3+ + Fe0, which produces metallic Fe.
View in article
Rohrbach, A., Ghosh, S., Schmidt, M.W., Wijbrans, C.H., Klemme, S. (2014) The stability of Fe–Ni carbides in the Earth's mantle: Evidence for a low Fe–Ni–C melt fraction in the deep mantle. Earth and Planetary Science Letters 388, 211-221.
 Show in context
Show in context
Partial melting of the deep mantle with δ15N of −5 ‰, in the presence of 0.5 to 1.5 wt. % Fe-rich metal (Rohrbach et al., 2014), may therefore generate OIBs with very slightly positive δ15N values; however, generating OIBs with δ15N up to +8 ‰ may need the source region δ15N > +0 ‰.
View in article
Roskosz, M., Bouhifd, M., Jephcoat, A., Marty, B., Mysen, B. (2013) Nitrogen solubility in molten metal and silicate at high pressure and temperature. Geochimica et Cosmochimica Acta 121, 15-28.
 Show in context
Show in context
A significant fraction of nitrogen may have been segregated into the core during core-mantle separation (Roskosz et al., 2013).
View in article
However, considering that nitrogen solubility in reduced silicate melt ranges up to a few wt. % (Kadik et al., 2011, 2013; Roskosz et al., 2013) and only a few hundred ppm nitrogen was present in our silicate melt, partial crystallisation during quench may not have caused exsolution of nitrogen.
View in article
Figure 1 [...] The published DNmetal/silicate data (Kadik et al., 2011, 2013; Roskosz et al., 2013) are plotted for comparison.
View in article
Rubie, D.C., Jacobson, S.A., Morbidelli, A., O’Brien, D.P., Young, E.D., de Vries, J., Nimmo, F., Palme, H., Frost, D.J. (2015) Accretion and differentiation of the terrestrial planets with implications for the compositions of early-formed Solar System bodies and accretion of water. Icarus 248, 89-108.
 Show in context
Show in context
Such a hybrid model (Rubie et al., 2015) assumes that during the early stages of accretion, the impacting objects are relatively small and their metal phase sequentially extracts nitrogen from the magma ocean according to a Rayleigh model; however, towards the end of accretion, large, differentiated planetesimals collide with the growing Earth.
View in article
According to Rubie et al. (2015), 80-100 % of the core-forming metal may have equilibrated with the silicate magma ocean according to the Rayleigh model.
View in article
Shen, Y., Solomon, S.C., Bjarnason, I.T., Wolfe, C.J. (1998) Seismic evidence for a lower-mantle origin of the Iceland plume. Nature 395, 62-65.
 Show in context
Show in context
The oceanic island basalts (OIBs), thought to be derived from the lower mantle (e.g., Shen et al., 1998; Stuart et al., 2003), have δ15N of −2 to +8 ‰, with a mean value of about +3 ‰ (Dauphas and Marty, 1999; Marty and Dauphas, 2003).
View in article
Stuart, F.M., Lass-Evans, S., Fitton, J.G., Ellam, R.M. (2003) High 3He/4He ratios in picritic basalts from Baffin Island and the role of a mixed reservoir in mantle plumes. Nature 424, 57-59.
 Show in context
Show in context
The oceanic island basalts (OIBs), thought to be derived from the lower mantle (e.g., Shen et al., 1998; Stuart et al., 2003), have δ15N of −2 to +8 ‰, with a mean value of about +3 ‰ (Dauphas and Marty, 1999; Marty and Dauphas, 2003).
View in article
Thomazo, C., Papineau, D. (2013) Biogeochemical cycling of nitrogen on the early Earth. Elements 9, 345-351.
 Show in context
Show in context
The δ15N value of the organic matter and metasediments is overall positive, and the δ15N value of Earth’s surface (crust + atmosphere) is approximately +2 ‰ (Cartigny and Marty, 2013; Thomazo and Papineau, 2013).
View in article
Trinquier, A., Birck, J., Allègre, C.J. (2007) Widespread 54Cr heterogeneity in the inner solar system. The Astrophysical Journal 655, 1179.
 Show in context
Show in context
These results are also consistent with several other isotopic systems that point towards enstatite chondrites as a main source of the material from which the Earth formed (Javoy, 1997; Trinquier et al., 2007; Regelous et al., 2008; Javoy et al., 2010).
View in article
Wood, B.J., Walter, M.J., Wade, J. (2006) Accretion of the Earth and segregation of its core. Nature 441, 825-832.
 Show in context
Show in context
The second endmember mode is the continuous core formation model (Wood et al., 2006, 2013).
View in article
Wood, B.J., Li, J., Shahar, A. (2013) Carbon in the core: its influence on the properties of core and mantle. Reviews in Mineralogy and Geochememistry 75, 231-250.
 Show in context
Show in context
The second endmember mode is the continuous core formation model (Wood et al., 2006, 2013).
View in article
The fractionation of light element isotopes in this model can best be described by the Rayleigh distillation model (Wood et al., 2013; Horita and Polyakov, 2015): δ15Nmantle = δ15NBE + Δ15Nmetal-silicate × lnƒNmantle where ƒNmantle is the remaining fraction of nitrogen in the silicate mantle just after complete core-mantle separation.
View in article
top
Supplementary Information
Methods
Starting materials for high-pressure experiments included high-purity iron powder, ammonium nitrate (NH4NO3), and a synthetic silicate glass with a chemical composition similar to that of the global MORB. A mixture of iron powder and finely-ground silicate glass was loaded together with about 0.1 to 1.5 wt. % ammonium nitrate into a graphite-lined platinum capsule. Experiments were conducted at 1.5 to 7.0 GPa and 1600 to 1800 °C in a piston cylinder or multi-anvil high-pressure apparatus, such that only liquid Fe-rich metal and basaltic melt were coexisting in the sample charge. Experiments were quenched to room temperature within a few seconds after about 90 mins. The coexisting Fe-rich metal and silicate of the recovered samples were carefully separated for analyses of nitrogen concentration and isotopic composition, using a static gas mass spectrometer.
Individual chips of glass and metal weighting a few micrograms to a few milligrams were loaded in a laser heating cell consisting of a metal sample holder covered by a ZnSe window. The cell was baked at about 100 °C and pumped under high vacuum (10-8-10-9 mbar) for several days. Individual samples were then incrementally heated in static vacuum using a CO2 laser (wavelength = 10.6 μm). Evolved gases including N compounds were purified for carbon and hydrogen bearing species using a combination of a CuO furnace, Pt catalyst and cold trap held just above the liquid N2 temperature. Purified N2 was then introduced in a static mass spectrometer where masses 28 and 29 were analysed on a Faraday cup detector and masses 29 and 30 were counted on an electron multiplier (Humbert et al., 2000
Humbert, F., Libourel, G., France-Lanord, C., Zimmermann, L., Marty, B. (2000) CO2-laser extraction-static mass spectrometry analysis of ultra-low concentrations of nitrogen in silicates. Geostandards Newsletter 24, 255-260.
). Potential CO contamination, checked with the measured 29/30 ratio, was found to be always negligible. The abundances of nitrogen and the 15N/14N ratios were then computed from the data and from runs with our in-house N2 standard (purified atmospheric N2). Blanks were found to be less than 0.1 % of the signals for all runs. Errors on the isotopic ratios are computed from the standard deviation of in-house N2 standard runs and from the internal precision of the respective sample runs.Nitrogen metal/silicate partition coefficients DNmetal/silicate were calculated according to DNmetal/silicate = CNmetal/CNsilicate , where CNmetal and CNsilicate are the nitrogen concentrations in the liquid Fe-rich metal and silicate melt, respectively. Nitrogen metal/silicate isotope fractionation Δ15Nmetal-silicate was calculated according to Δ15Nmetal-silicate = Δ15Nmetal - Δ15Nsilicate, where Δ15Nmetal and Δ15Nsilicate are the nitrogen isotopic composition of the liquid Fe-rich metal and silicate melt, respectively.
Table S-1 Major and minor element contents in silicate melt and liquid Fe-rich metal (in wt. %).
| silicate | metal | ||||||||||||||
| Exp.ID | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MgO | CaO | Na2O | K2O | Total | Fe | Pt | C | O | Total |
| YS-1 | 43.4 | 1.3 | 12.9 | 0.0 | 25.4 | 6.4 | 9.3 | 2.2 | 0.2 | 101.1 | 79.7 | 14.2 | 6.2 | 0.1 | 100.2 |
| 1-σ | 0.7 | 0.1 | 0.2 | 0.0 | 1.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.4 | 1.7 | 2.1 | 0.6 | 0.1 | 0.6 |
| A-659 | 47.4 | 1.4 | 14.6 | 0.0 | 15.4 | 6.9 | 10.2 | 2.6 | 0.2 | 98.9 | 92.4 | 0.0 | 10.5 | 0.1 | 103.1 |
| 1-σ | 0.2 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.3 | 1.9 | 0.0 | 4.5 | 0.0 | 1.8 |
| YS-5 | 40.3 | 1.1 | 12.3 | 0.0 | 30.4 | 6.0 | 8.9 | 1.8 | 0.0 | 101.0 | 76.0 | 17.7 | 6.3 | 0.4 | 100.4 |
| 1-σ | 1.5 | 0.2 | 0.5 | 0.0 | 2.4 | 0.3 | 0.6 | 0.2 | 0.0 | 0.6 | 1.8 | 1.8 | 0.5 | 0.3 | 0.7 |
| YS-2 | 43.5 | 1.3 | 13.1 | 0.0 | 25.0 | 6.3 | 9.3 | 2.4 | 0.2 | 101.1 | 77.5 | 16.1 | 6.8 | 0.2 | 100.5 |
| 1-σ | 0.7 | 0.1 | 0.3 | 0.0 | 1.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 3.3 | 4.8 | 0.9 | 0.5 | 0.7 |
All the major and minor elements were measured by electron microprobe.
1-σ is the standard deviation based on replicate analyses of 10-20 spots.
The Pt in the metal is due to contamination by the Pt capsule used.
Supplementary Information References
Humbert, F., Libourel, G., France-Lanord, C., Zimmermann, L., Marty, B. (2000) CO2-laser extraction-static mass spectrometry analysis of ultra-low concentrations of nitrogen in silicates. Geostandards Newsletter 24, 255-260.
 Show in context
Show in context
Purified N2 was then introduced in a static mass spectrometer where masses 28 and 29 were analysed on a Faraday cup detector and masses 29 and 30 were counted on an electron multiplier (Humbert et al., 2000).
View in Supplementary Information
Figures and Tables
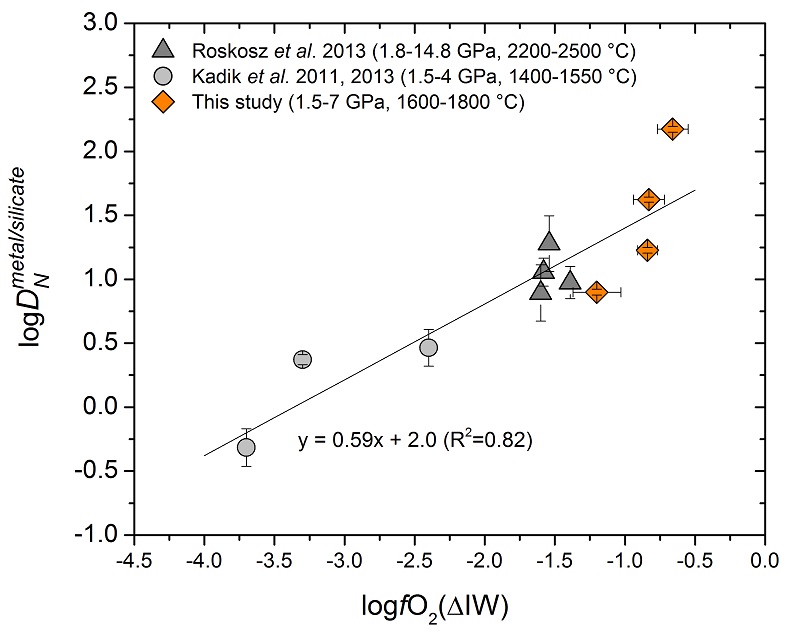
Figure 1 Nitrogen partition coefficients between liquid Fe-rich metal and silicate melt (DNmetal/silicate). The published DNmetal/silicate data (Kadik et al., 2011
Kadik, A., Kurovskaya, N., Ignat’ev, Y., Kononkova, N., Koltashev, V., Plotnichenko, V. (2011) Influence of oxygen fugacity on the solubility of nitrogen, carbon, and hydrogen in FeO-N2O-SiO2-Al2O3 melts in equilibrium with metallic iron at 1.5 GPa and 1400 °C. Geochemistry International 49, 429-438.
, 2013Kadik, A., Litvin, Y., Koltashev, V., Kryukova, E., Plotnichenko, V., Tsekhonya, T., Kononkova, N. (2013) Solution behavior of reduced N–H–O volatiles in FeO–Na2O–SiO2–Al2O3 melt equilibrated with molten Fe alloy at high pressure and temperature. Physics of the Earth and Planetary Interiors 214, 14-24.
; Roskosz et al., 2013Roskosz, M., Bouhifd, M., Jephcoat, A., Marty, B., Mysen, B. (2013) Nitrogen solubility in molten metal and silicate at high pressure and temperature. Geochimica et Cosmochimica Acta 121, 15-28.
) are plotted for comparison. DNmetal/silicate is a function of oxygen fugacity, regardless of pressure or temperature. The slope of the trend line is moderately lower than 0.75 expected based on Eq. 1, which could be due to a small fraction of nitrogen present as N2 in the silicate melt. Errors of DNmetal/silicate in this study are at 95 % confidence interval.Table 1 Experimental results on nitrogen partitioning and isotope fractionation between liquid Fe-rich metal and silicate melt.
| Exp. ID | P | T | Run duration | alogƒO2(ΔIW) | N con. in metal | N con. in silicate | δ15N of metal | δ15N of silicate | DNmetal/silicate | Δ15Nmetal−silicate | ||||||||||||||
| GPa | °C | mins | mol/g | mol/g | ||||||||||||||||||||
| Piston Cylinder Experiments | ||||||||||||||||||||||||
| Y-1 | 2.0 | 1600 | 120 | -0.84 | ± | 0.07 | 3.7E-04 | ± | 1.3E-05 | 2.2E-05 | ± | 7.9E-07 | 9.2 | ± | 1.6 | 13.3 | ± | 1.8 | 17 | ± | 1 | -4.1 | ± | 2.4 |
| A-659 | 1.5 | 1600 | 90 | -1.2 | ± | 0.17 | 4.9E-04 | ± | 1.8E-05 | 6.2E-05 | ± | 2.3E-06 | -1.1 | ± | 2.0 | 2.6 | ± | 1.6 | 8 | ± | 0.4 | -3.8 | ± | 2.5 |
| A-666 | 2.5 | 1700 | 30 | n.d. | 2.6E-06 | ± | 9.4E-08 | 3.4E-06 | ± | 1.3E-07 | -3.3 | ± | 2.4 | -2.2 | ± | 1.4 | 1 | ± | 0.04 | -1.1 | ± | 2.8 | ||
| Multi-Anvil Experiments | ||||||||||||||||||||||||
| YS-5 | 5.0 | 1700 | 90 | -0.66 | ± | 0.11 | 2.4E-03 | ± | 8.7E-05 | 1.6E-05 | ± | 5.8E-07 | 1.7 | ± | 1.5 | 7.2 | ± | 1.7 | 149 | ± | 8 | -5.5 | ± | 2.3 |
| YS-1 | 7.0 | 1700 | 90 | n.d. | 7.2E-04 | ± | 2.6E-05 | 3.6E-05 | ± | 1.3E-06 | -0.6 | ± | 1.9 | 1.4 | ± | 1.7 | 20 | ± | 1 | -2.0 | ± | 2.5 | ||
| YS-2 | 7.0 | 1800 | 90 | -0.83 | ± | 0.11 | 1.0E-03 | ± | 3.8E-05 | 2.5E-05 | ± | 6.8E-07 | -2.0 | ± | 2.1 | 2.6 | ± | 1.5 | 42 | ± | 2 | -4.6 | ± | 2.6 |
a: The logƒO2(ΔIW) was calcualted using this equilibrium: FeO (silicate melt) = Fe (liquid metal) + 1/2O2, from which the ƒO2 of the experiment relative to ƒO2 of the iron-wüstite buffer (IW) can be defined as: ΔIW = 2 log (XFeOγFeO/XFeγFe), XFeO and XFe are the mole fractions of FeO in silicate melt and Fe in liquid metal, respectively;
γFeO and γFe are the activity coefficients of FeO in silicate melt and Fe in liquid metal, respectively.
Calculation of logƒO2(ΔIW) was performed assuming γFeO = 1.2 in the silicate melt (O'Neill and Eggins, 2002
O'Neill, H.S.C., Eggins, S.M. (2002) The effect of melt composition on trace element partitioning: an experimental investigation of the activity coefficients of FeO, NiO, CoO, MoO2 and MoO3 in silicate melts. Chemical Geology 186, 151-181.
) and ideal solution of liquid Fe-C-Pt-N metal (γFe = 1).
For major element compositions of silicate melt and liquid metal, see Supplementary Table 1.
n.d.: not determined.
Errors for DNmetal/silicate and Δ15Nmetal-silicate are at 95 % confidence interval.
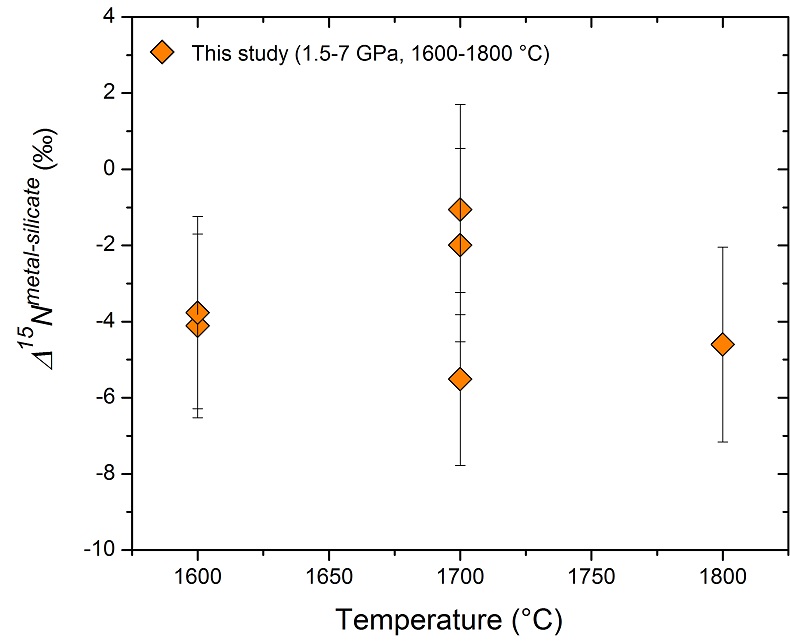
Figure 2 Nitrogen isotope fractionation between liquid Fe-rich metal and silicate melt (Δ15Nmetal-silicate). Temperature has a very limited effect on Δ15Nmetal-silicate in the P-T range studied. Errors of Δ15Nmetal-silicate are at 95 % confidence interval.
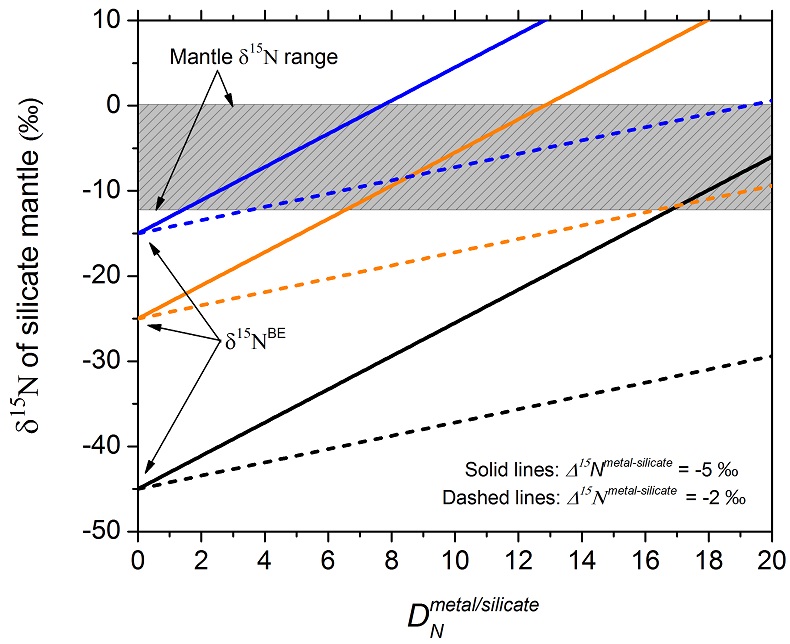
Figure 3 The modelled δ15N value of the silicate mantle as a function of DNmetal/silicate, just after complete core-mantle separation, using the Rayleigh distillation model (Eq. 5) and the relationship between ƒNmantle and DNmetal/silicate (Eqs. 6 and 7). Three different δ15NBE values are used: the lowest value (−45 ‰) is the lowest value observed so far for Earth’s mantle and enstatite chondrites (Grady et al., 1986
Grady, M.M., Wright, I., Carr, L., Pillinger, C. (1986) Compositional differences in enstatite chondrites based on carbon and nitrogen stable isotope measurements. Geochimica et Cosmochimica Acta 50, 2799-2813.
; Palot et al., 2012Palot, M., Cartigny, P., Harris, J., Kaminsky, F., Stachel, T. (2012) Evidence for deep mantle convection and primordial heterogeneity from nitrogen and carbon stable isotopes in diamond. Earth and Planetary Science Letters 357, 179-193.
); the values of −25 ‰ and −15 ‰ correspond to the average and highest δ15N values of enstatite chondrites (Grady et al., 1986Grady, M.M., Wright, I., Carr, L., Pillinger, C. (1986) Compositional differences in enstatite chondrites based on carbon and nitrogen stable isotope measurements. Geochimica et Cosmochimica Acta 50, 2799-2813.
), respectively. Two different values of −2 ‰ and −5 ‰ are used for the dashed lines and solid lines, respectively. The gray box represents the main range of δ15N values observed for the silicate mantle (Marty and Dauphas, 2003Marty, B., Dauphas, N. (2003) The nitrogen record of crust-mantle interaction and mantle convection from Archean to Present. Earth and Planetary Science Letters 206, 397-410.
; Palot et al., 2012Palot, M., Cartigny, P., Harris, J., Kaminsky, F., Stachel, T. (2012) Evidence for deep mantle convection and primordial heterogeneity from nitrogen and carbon stable isotopes in diamond. Earth and Planetary Science Letters 357, 179-193.
; Cartigny and Marty, 2013Cartigny, P., Marty, B. (2013) Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere–crust–mantle connection. Elements 9, 359-366.
), which may be produced from the average nitrogen isotopic composition of enstatite chondrites by core-mantle separation with DNmetal/silicate between 5 and 20.Supplementary Figures and Tables
Table S-1 Major and minor element contents in silicate melt and liquid Fe-rich metal (in wt. %).
| silicate | metal | ||||||||||||||
| Exp.ID | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MgO | CaO | Na2O | K2O | Total | Fe | Pt | C | O | Total |
| YS-1 | 43.4 | 1.3 | 12.9 | 0.0 | 25.4 | 6.4 | 9.3 | 2.2 | 0.2 | 101.1 | 79.7 | 14.2 | 6.2 | 0.1 | 100.2 |
| 1-σ | 0.7 | 0.1 | 0.2 | 0.0 | 1.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.4 | 1.7 | 2.1 | 0.6 | 0.1 | 0.6 |
| A-659 | 47.4 | 1.4 | 14.6 | 0.0 | 15.4 | 6.9 | 10.2 | 2.6 | 0.2 | 98.9 | 92.4 | 0.0 | 10.5 | 0.1 | 103.1 |
| 1-σ | 0.2 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.3 | 1.9 | 0.0 | 4.5 | 0.0 | 1.8 |
| YS-5 | 40.3 | 1.1 | 12.3 | 0.0 | 30.4 | 6.0 | 8.9 | 1.8 | 0.0 | 101.0 | 76.0 | 17.7 | 6.3 | 0.4 | 100.4 |
| 1-σ | 1.5 | 0.2 | 0.5 | 0.0 | 2.4 | 0.3 | 0.6 | 0.2 | 0.0 | 0.6 | 1.8 | 1.8 | 0.5 | 0.3 | 0.7 |
| YS-2 | 43.5 | 1.3 | 13.1 | 0.0 | 25.0 | 6.3 | 9.3 | 2.4 | 0.2 | 101.1 | 77.5 | 16.1 | 6.8 | 0.2 | 100.5 |
| 1-σ | 0.7 | 0.1 | 0.3 | 0.0 | 1.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 3.3 | 4.8 | 0.9 | 0.5 | 0.7 |
All the major and minor elements were measured by electron microprobe.
1-σ is the standard deviation based on replicate analyses of 10-20 spots.
The Pt in the metal is due to contamination by the Pt capsule used.